
Predict (to 3 sig figs) the isochoric specific heat of gaseous sf6 (molar mass 146.06 g/mol) at 1200 k, assuming that at this temperature all translational, rotational and vibrational degrees of freedom are accessible, and assuming no electronic degrees of freedom are accessible at all. do you need to assume ideal fully gas behavior?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1



Chemistry, 22.06.2019 16:00
Answer asap : ( a. how does mucus prevent the entry of pathogens? b. describe two ways white blood cells protect us from pathogens.
Answers: 1
You know the right answer?
Predict (to 3 sig figs) the isochoric specific heat of gaseous sf6 (molar mass 146.06 g/mol) at 1200...
Questions

Social Studies, 30.10.2019 17:31




Business, 30.10.2019 17:31

English, 30.10.2019 17:31



Social Studies, 30.10.2019 17:31



Mathematics, 30.10.2019 17:31

Mathematics, 30.10.2019 17:31

Mathematics, 30.10.2019 17:31

History, 30.10.2019 17:31


History, 30.10.2019 17:31

Mathematics, 30.10.2019 17:31

Mathematics, 30.10.2019 17:31

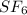 molecule is a non-linear molecule. Therefore, its isochoric heat capacity will be as follows.
molecule is a non-linear molecule. Therefore, its isochoric heat capacity will be as follows.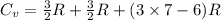
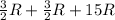
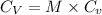
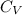 = molar heat capacity
= molar heat capacity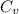 = specific heat
= specific heat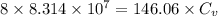
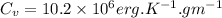
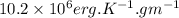 .
.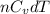
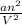 " has to be added here.
" has to be added here.


