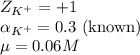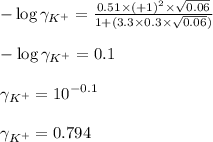Chemistry, 10.10.2019 04:30 ayoismeisjjjjuan
Calculate the activity coefficients for the following conditions:
a. cu2+ in a 0.01 m nacl solution
b. k+in a 0.025m hcl solution
c. k+in a 0.02 k2so4 solution

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2


Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
You know the right answer?
Calculate the activity coefficients for the following conditions:
a. cu2+ in a 0.01 m n...
a. cu2+ in a 0.01 m n...
Questions


Mathematics, 18.06.2020 19:57

Physics, 18.06.2020 19:57

Mathematics, 18.06.2020 19:57

Biology, 18.06.2020 19:57


History, 18.06.2020 19:57


Mathematics, 18.06.2020 19:57

Mathematics, 18.06.2020 19:57


Chemistry, 18.06.2020 19:57


Mathematics, 18.06.2020 19:57




Mathematics, 18.06.2020 19:57


Chemistry, 18.06.2020 19:57

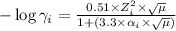 ........(1)
........(1)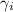 = activity coefficient of ion
= activity coefficient of ion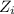 = charge of the ion
= charge of the ion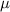 = ionic strength of solution
= ionic strength of solution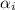 = diameter of the ion in nm
= diameter of the ion in nm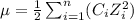 ......(2)
......(2)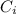 = concentration of i-th ions
= concentration of i-th ions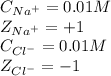
![\mu=\frac{1}{2}[(0.01\times (+1)^2)+(0.01\times (-1)^2)]\\\\\mu=0.01M](/tpl/images/0305/8447/4f4d0.png)
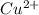 ion in the solution by using equation 1:
ion in the solution by using equation 1: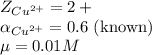
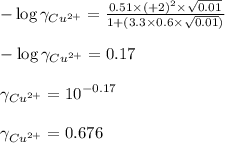
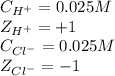
![\mu=\frac{1}{2}[(0.025\times (+1)^2)+(0.025\times (-1)^2)]\\\\\mu=0.025M](/tpl/images/0305/8447/8d055.png)
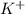 ion in the solution by using equation 1:
ion in the solution by using equation 1: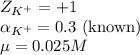
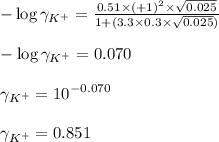
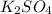 solution:
solution: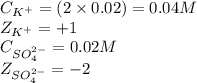
![\mu=\frac{1}{2}[(0.04\times (+1)^2)+(0.02\times (-2)^2)]\\\\\mu=0.06M](/tpl/images/0305/8447/637cf.png)