
Ahypothetical one-electron atom has these energy levels:
e6 = -2 x 10-19 j
e5 = -7 x 10-19 j
e4 = -11 x 10-19 j
e3 = -15 x 10-19 j
e2 = -17 x 10-19 j
e1 = -20 x 10-19 j
(this is not an actual atom that exists. according to the bohr model, energies should follow the expression discussed in section 6.2 of silberberg. this exercise prepares you for calculations like questions 6 and 7 of problem set 1.)
(a) if the electron is initially in the n = 4 level, what is the shortest wavelength of radiation that could be emitted? provide your answer to one significant figure: -7 m.
(b) what is the ionization energy (in kj/mol) of the atom in its first excited state? provide your answer to two significant figures: 103 kj/mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 3

Chemistry, 23.06.2019 06:30
An engineer decides to use a slightly weaker material rather than a stronger material, since she knows that the stronger material can break suddenly. this is an example of what? a choosing a material that will show warning before it fails b using composite materials that combine strength c using a material for multiple applications d using design techniques that increase efficiency and reduce cost
Answers: 3
You know the right answer?
Ahypothetical one-electron atom has these energy levels:
e6 = -2 x 10-19 j
...
e6 = -2 x 10-19 j
...
Questions


Chemistry, 20.03.2020 06:41


Computers and Technology, 20.03.2020 06:41






History, 20.03.2020 06:42


Mathematics, 20.03.2020 06:43


Mathematics, 20.03.2020 06:43


Biology, 20.03.2020 06:43




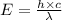
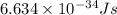
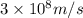
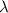 = Wavelength of the radiation
= Wavelength of the radiation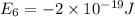
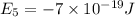
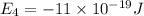
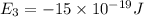
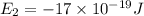
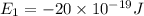
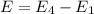
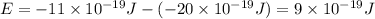
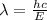
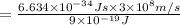
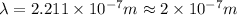
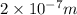 is the shortest wavelength of radiation that could be emitted.
is the shortest wavelength of radiation that could be emitted.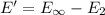
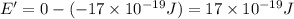
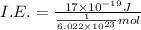
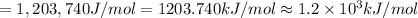
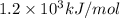 is the ionization energy of the atom in its first excited state.
is the ionization energy of the atom in its first excited state.

