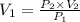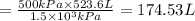Chemistry, 10.10.2019 05:20 ayoismeisjjjjuan
An empty balloon is connected to a tank containing helium at 1.5 mpa and 25 °c. a valve connecting the tank and the balloon is opened until the diameter of the spherical balloon reaches a diameter of 1 m. at this point, the pressure of he inside the balloon is 500 kpa. assuming the whole process to be isothermic, determine the minimum volume that the tank must have to inflate this balloon to its 1 m diameter

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2

Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1

Chemistry, 23.06.2019 01:30
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3
You know the right answer?
An empty balloon is connected to a tank containing helium at 1.5 mpa and 25 °c. a valve connecting t...
Questions

History, 14.04.2021 22:00

Mathematics, 14.04.2021 22:00






Arts, 14.04.2021 22:00

Mathematics, 14.04.2021 22:00



Mathematics, 14.04.2021 22:00




Mathematics, 14.04.2021 22:00

Mathematics, 14.04.2021 22:00


Advanced Placement (AP), 14.04.2021 22:00

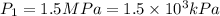
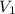
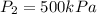
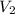

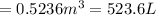
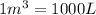
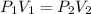 (Boyle's law)
(Boyle's law)