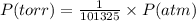Chemistry, 10.10.2019 05:20 hillarytrinh
495 cm3 of oxygen gas at 25 oc and 114,700 pa, and 877 cm3 of nitrogen gas 25.0 °c and 114,700 pa, are injected into an evacuated 536 cm3 flask.
a. find the number of moles of oxygen present prior to mixing the gases, assuming the temperature remains constant, and that oxygen is an ideal gas. (5pts)
b. find the number of moles of nitrogen present prior to mixing the gases, assuming the temperature remains constant and that nitrogen is an ideal gas. (5pts)
c. find the total pressure of oxygen and nitrogen in the flask (after the two gases are mixed together.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

You know the right answer?
495 cm3 of oxygen gas at 25 oc and 114,700 pa, and 877 cm3 of nitrogen gas 25.0 °c and 114,700 pa, a...
Questions




Chemistry, 04.12.2020 14:00

Mathematics, 04.12.2020 14:00

History, 04.12.2020 14:00

Computers and Technology, 04.12.2020 14:00

Mathematics, 04.12.2020 14:00

English, 04.12.2020 14:00

English, 04.12.2020 14:00


Social Studies, 04.12.2020 14:00


Mathematics, 04.12.2020 14:00



Mathematics, 04.12.2020 14:00

Mathematics, 04.12.2020 14:00

Social Studies, 04.12.2020 14:00

Mathematics, 04.12.2020 14:00