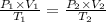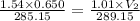Abubble of helium gas has a volume of 0.650 cm3 near the bottom of a large aquarium where the pressure is 1.54 atm and the temperature is 12°c. determine the bubble’s volume upon rising near the top where the pressure is 1.01 atm and 16°c. assume that the number of moles of helium remains constant and that the helium is an ideal gas. (13 pts)
important equations and constants 1 atm = 760 torr = 760 mmhg = 101,325 pa
1ml = 1cm3
pv = nrt
p1v1 = p2v2
v1/t1 = v2/t2
v1/n1 = v2/n2
ptotal = p1 + p2 + p3 + ….
(p1v1)/(n1t1) = (p2v2)/(n2t2)
r = 0.08206 l atm/mol k

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
Abubble of helium gas has a volume of 0.650 cm3 near the bottom of a large aquarium where the pressu...
Questions




English, 21.09.2019 08:50




Biology, 21.09.2019 08:50

Biology, 21.09.2019 08:50

Spanish, 21.09.2019 08:50

History, 21.09.2019 08:50



Mathematics, 21.09.2019 08:50


Mathematics, 21.09.2019 08:50

Mathematics, 21.09.2019 08:50

History, 21.09.2019 08:50

History, 21.09.2019 08:50

Physics, 21.09.2019 08:50