

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 00:00
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2


Chemistry, 23.06.2019 04:00
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2

Chemistry, 23.06.2019 05:20
Explain how global warming could have affected yellowstone frog and salamander habitat's, resulting in changes in the populations of these species
Answers: 2
You know the right answer?
What is the percent by mass of oxygen in a gaseous mixture whose molar composition is 0.500 % co2 an...
Questions

















Spanish, 13.03.2020 22:48


Mathematics, 13.03.2020 22:48

Mathematics, 13.03.2020 22:48

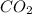 = 0.500 %
= 0.500 %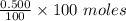 = 0.5 moles
= 0.5 moles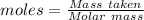
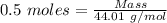
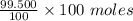 = 99.500 moles
= 99.500 moles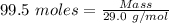
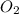 = 21 % of air
= 21 % of air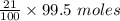 = 20.895 moles
= 20.895 moles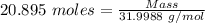
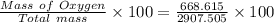 = 23.0 %
= 23.0 %

