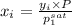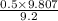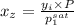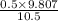Chemistry, 10.10.2019 05:30 aleahnew36
Aclosed system contains an equimolar mixture of n-pentane and isopentane. suppose the system is initially all liquid at 120°c and a high pressure, and the pressure is gradually reduced at a constant temperature. estimate the pressures at which the first bubble of vapor forms and at which the last drop of liquid evaporates. also calculate the liquid and vapor compositions (mole fractions) at those two conditions.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1


Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1
You know the right answer?
Aclosed system contains an equimolar mixture of n-pentane and isopentane. suppose the system is init...
Questions

Social Studies, 15.01.2021 19:20





Mathematics, 15.01.2021 19:20

Mathematics, 15.01.2021 19:20


History, 15.01.2021 19:20


Mathematics, 15.01.2021 19:20

Geography, 15.01.2021 19:20

History, 15.01.2021 19:20


History, 15.01.2021 19:20


Mathematics, 15.01.2021 19:20

Mathematics, 15.01.2021 19:20

Advanced Placement (AP), 15.01.2021 19:20

English, 15.01.2021 19:20

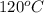 = (120 + 273.15)K = 393.15 K,
= (120 + 273.15)K = 393.15 K, 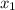 = 0.5 and
= 0.5 and 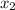 = 0.5
= 0.5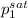 (393.15 K) = 9.2 bar
(393.15 K) = 9.2 bar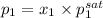
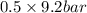
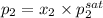
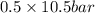
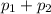
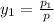
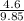
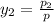
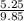
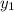 = 0.5,
= 0.5, 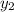 = 0.5
= 0.5 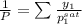
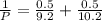
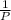 = 0.101966
= 0.101966 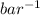
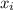 and its formula is as follows.
and its formula is as follows.