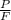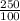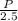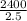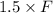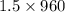Chemistry, 10.10.2019 05:30 yhbgvfcd6677
An aqueous solution of sodium hydroxide contains 15% naoh by mass. it is desired to produce an 5% naoh solution by diluting a stream of the 15% solution with a stream of pure water. calculate the following: a) the ratios (g h, o/g feed solution) and (g product / g feed solution). b) determine the feed rates of 15% solution and diluting water needed to produce 2500 ib./min of the 5% solution.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1


Chemistry, 23.06.2019 12:30
Idid a lab for chemistry where we put nails in a copper (ii) chloride solution. 1. why did the reaction stop? which reactant was used up? how do you know? 2. describe what was happening to the atoms of iron and copper during the reaction. what is this type of reaction called? 3. what would happen to the ratio of copper to iron if you had placed more nails in the beaker? if you had let the reaction go for less time? 4. what is the accepted ratio of copper atoms to iron atoms in this reaction? account for differences between your experimental value and the accepted value. write the balanced equation for the reaction.
Answers: 2
You know the right answer?
An aqueous solution of sodium hydroxide contains 15% naoh by mass. it is desired to produce an 5% na...
Questions


Biology, 27.02.2020 18:23

Social Studies, 27.02.2020 18:23





Mathematics, 27.02.2020 18:23

History, 27.02.2020 18:23











Mathematics, 27.02.2020 18:24

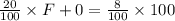
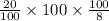
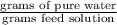 =
= 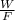
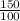
 =
=