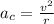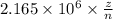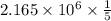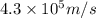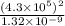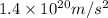Consider the hydrogen atom as described by the bohr model. the nucleus of the hydrogen atom is a single proton. the electron rotates in a circular orbit about this nucleus. in the n = 5, orbit the electron is 1.32 10-9 m from the nucleus and it rotates with an angular speed of 3.30 1014 rad/s. determine the electron's centripetal acceleration in m/s2.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which compounds have the empirical formula ch2o? a.c2h4o2 b.c3h6o3 c.ch2o2 d.c5h10o5 e.c6h12o6
Answers: 3

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

You know the right answer?
Consider the hydrogen atom as described by the bohr model. the nucleus of the hydrogen atom is a sin...
Questions














Geography, 18.11.2019 22:31






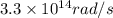 .
.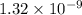 m
m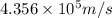
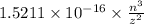 sec
sec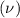 =
= 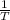
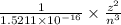
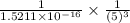
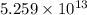 Hz
Hz