
As you move down through the family of alkali metals, the first ionization energy because
a) increases; the atomic radii increase.
b) increases; the atomic radii decrease.
c) decreases; the atomic radii increase.
d) remains constant; all the elements lose one electron.
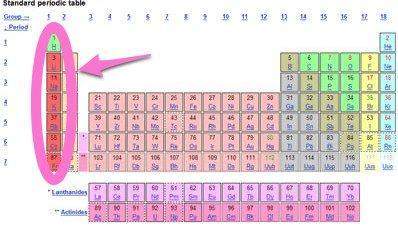

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2

Chemistry, 23.06.2019 00:30
Fred is studying a substance that is made out of only one element. this means that
Answers: 1

Chemistry, 23.06.2019 00:30
What is the chemical formula of magnesium bromide? a. mgbr2 b. mgbr c. mg2br2 d. mg2br
Answers: 3
You know the right answer?
As you move down through the family of alkali metals, the first ionization energy because
Questions




Mathematics, 12.03.2021 21:40


Chemistry, 12.03.2021 21:40

Mathematics, 12.03.2021 21:40

Mathematics, 12.03.2021 21:40



Spanish, 12.03.2021 21:40




Physics, 12.03.2021 21:40

Mathematics, 12.03.2021 21:40

Mathematics, 12.03.2021 21:40

History, 12.03.2021 21:40

Mathematics, 12.03.2021 21:40



