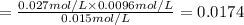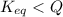At 1173 k, keq = 0.0108 for the following reaction: caco3(s) ⇄ cao(s) + co2(g) the reaction takes place in a 10.0 l vessel at 1173 k. if a mixture of 15.0 g caco3, 15.0 g cao, and 4.25 g co2 is allowed to approach equilibrium, what will happen to the amount of caco3? group of answer choices
- it will remain the same
- it will increase
- not enough information is provided to answer this question
- it will decrease

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:00
When a comet collides with earth, it adds material to our planet and causes great damage. therefore, a collision like this is a a. destructive force b. constructive force c. geologic process and event d. constructive and destructive force
Answers: 1

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1

Chemistry, 23.06.2019 06:10
2. what two items do autotrophs take from the environment to produce their food? 3. what are the two items that are released during transpiration from leaves? 4. what are the two membranes of the system? a.what are the two stages of photosynthesis? what are the two parts of photosynthesis?
Answers: 2

Chemistry, 23.06.2019 15:30
Which answer below correctly identifies the type of change and the explanation when magnesium comes into contact with hydrochloric acid
Answers: 1
You know the right answer?
At 1173 k, keq = 0.0108 for the following reaction: caco3(s) ⇄ cao(s) + co2(g) the reaction takes p...
Questions

Arts, 29.12.2020 21:40


Biology, 29.12.2020 21:40

Mathematics, 29.12.2020 21:40



Mathematics, 29.12.2020 21:40

Mathematics, 29.12.2020 21:40



Social Studies, 29.12.2020 21:40

World Languages, 29.12.2020 21:40


Computers and Technology, 29.12.2020 21:40


History, 29.12.2020 21:40


Mathematics, 29.12.2020 21:40


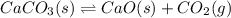
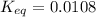
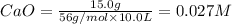
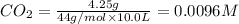
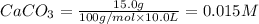
![Q=\frac{[CaO][CO_2]}{[CaCO_3]}](/tpl/images/0308/1118/76f7b.png)