
Chemistry, 11.10.2019 00:00 jackfrost5
Astandard solution of fescn2+ is prepared by combining 9.0 ml of 0.20 m fe(no3)3 with 1.0 ml of 0.0020 m kscn . the standard solution had an absorbance of 0.480 . fe3+(aq)+scn−(aq)↽−−⇀fescn2+(aq) a trial solution was made in a similar manner, but with a more dilute fe(no3)3 reagent. the initial scn− concentration, immediately after mixing, was 0.00050 m . this trial solution had absorbance of 0.220 . what is the equilibrium concentration of scn− in the trial solution?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1
You know the right answer?
Astandard solution of fescn2+ is prepared by combining 9.0 ml of 0.20 m fe(no3)3 with 1.0 ml of 0.00...
Questions



English, 07.06.2020 00:03

Mathematics, 07.06.2020 00:03



Mathematics, 07.06.2020 00:03

Mathematics, 07.06.2020 00:03


Social Studies, 07.06.2020 00:03






Mathematics, 07.06.2020 00:03


Mathematics, 07.06.2020 00:03


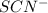 in the trial solution is
in the trial solution is 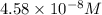
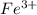 and
and 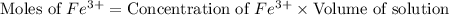
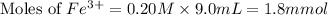
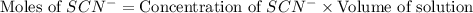
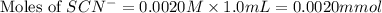
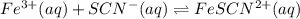
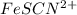
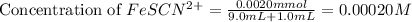

 = molar absorptivity coefficient
= molar absorptivity coefficient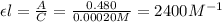
![[SCN^-]_{eqm}=[SCN^-]_{initial}-[FeSCN^{2+}]](/tpl/images/0308/4929/92d10.png)
![[SCN^-]_{initial}](/tpl/images/0308/4929/bc58d.png) = 0.00050 M
= 0.00050 M![[FeSCN^{2+}]](/tpl/images/0308/4929/797d4.png) .
.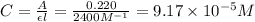
![[SCN^-]_{eqm}=(0.00050M)-(9.17\times 10^{-5}M)](/tpl/images/0308/4929/c2881.png)
![[SCN^-]_{eqm}=4.58\times 10^{-8}M](/tpl/images/0308/4929/1e6f2.png)


