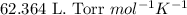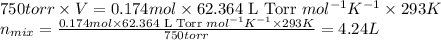Some commercial drain cleaners contain two components: sodium hydroxide and aluminum in the form of a powder. when the mixture is poured down a clogged drain, the following redox reactions occurs: 2naoh(aq) + 2 al(s) + 6h2o(l) → 2naal(oh)4(aq) + 3 h2(g) the heat generated in this reaction melt away grese and the hydrogen gas released stirs up the solids clagging the drain. calculate the volume hydrogen gas formed at 20. ºc and 750. torr if 3.12 g of al is treated with excess naoh.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1
You know the right answer?
Some commercial drain cleaners contain two components: sodium hydroxide and aluminum in the form of...
Questions

English, 12.12.2020 16:10



Mathematics, 12.12.2020 16:10

Mathematics, 12.12.2020 16:10




English, 12.12.2020 16:10

French, 12.12.2020 16:10

Mathematics, 12.12.2020 16:10

Mathematics, 12.12.2020 16:10



Engineering, 12.12.2020 16:10

Mathematics, 12.12.2020 16:10

Mathematics, 12.12.2020 16:10



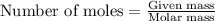
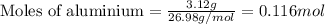
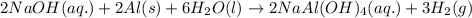
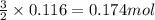 of hydrogen gas
of hydrogen gas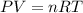
![20^oC=[20+273]K=293K](/tpl/images/0308/3868/3b5d4.png)