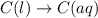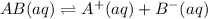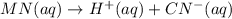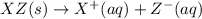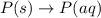Chemistry, 11.10.2019 00:10 look26goingjbgy
Each of the following reactions shows a solute dissolved in water. classify each solute as a strong electrolyte, a weak electrolyte, or a nonelectrolyte. c(l)→c(aq) ab(aq)⇌ a+(aq)+b−(aq) mn(aq)→m+(aq)+n−(aq) xz(s)→x+(aq)+z−(aq) p(s)→p(aq)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3


Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
You know the right answer?
Each of the following reactions shows a solute dissolved in water. classify each solute as a strong...
Questions


Mathematics, 28.09.2019 00:00


History, 28.09.2019 00:00




Mathematics, 28.09.2019 00:00


Mathematics, 28.09.2019 00:00

Mathematics, 28.09.2019 00:00

Mathematics, 28.09.2019 00:00




Mathematics, 28.09.2019 00:00



Mathematics, 28.09.2019 00:00

Social Studies, 28.09.2019 00:00