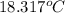Aquantity of 85.0 ml of 0.900 m hcl is mixed with 85.0 ml of 0.900 m koh in a constantpressure calorimeter that has a heat capacity of 325 j/°c. if the initial temperatures of both solutions are the same at 18.24°c, what is the final temperature of the mixed solution? the heat of neutralization is −56.2 kj/mol. assume the density and specific heat of the solutions are the same as those for water.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
219 grams of iron (iii) oxide reacts with excess carbon according to the reaction equation shown below. fe2o3 + c → fe + co2 after a scientist performs the chemical reaction they find the actual yield of iron to be 57.4 grams. calculate the percent yield of this chemical reaction.
Answers: 1


Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
Aquantity of 85.0 ml of 0.900 m hcl is mixed with 85.0 ml of 0.900 m koh in a constantpressure calor...
Questions

Computers and Technology, 26.11.2019 07:31














Computers and Technology, 26.11.2019 07:31





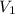 = 85.0 ml,
= 85.0 ml, 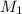 = 0.9 M
= 0.9 M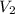 = 85.0 ml,
= 85.0 ml, 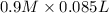 (as 1 L = 1000 ml so, 85 ml = 0.085 L)
(as 1 L = 1000 ml so, 85 ml = 0.085 L)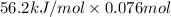
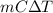
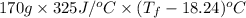
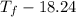
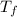 =
=