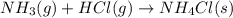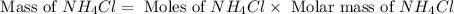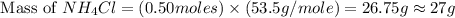For each of the following balanced chemical equations, calculate how many moles and how many grams of each product would be produced by the complete conversion of 0.50 mole of the reactant indicated in boldface. state clearly the mole ratio used for each conversion. a. nh3(g) 1 hcl(g) s nh4cl(s)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Drag each number to the correct location on the equation. each number can be used more than once, but not all numbers will be used. balance the equation with the coefficients. 2 3 4 5 kclo3 -> kcl + o2
Answers: 1

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

You know the right answer?
For each of the following balanced chemical equations, calculate how many moles and how many grams o...
Questions





Mathematics, 13.10.2020 19:01

Mathematics, 13.10.2020 19:01

History, 13.10.2020 19:01



History, 13.10.2020 19:01

English, 13.10.2020 19:01

Mathematics, 13.10.2020 19:01

Mathematics, 13.10.2020 19:01

Social Studies, 13.10.2020 19:01

English, 13.10.2020 19:01





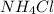 and mass of
and mass of 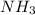 . So,
. So,