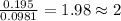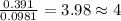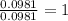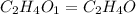Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1


Chemistry, 23.06.2019 07:30
How many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride
Answers: 1

Chemistry, 23.06.2019 08:00
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 3
You know the right answer?
The foul odor of rancid butter is due largely to butyric acid, a compound containing carbon, hydroge...
Questions

Mathematics, 11.12.2019 23:31


Mathematics, 11.12.2019 23:31

Mathematics, 11.12.2019 23:31

History, 11.12.2019 23:31

Social Studies, 11.12.2019 23:31

History, 11.12.2019 23:31






Mathematics, 11.12.2019 23:31

Physics, 11.12.2019 23:31






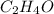
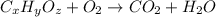
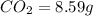
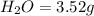
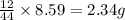 of carbon will be contained.
of carbon will be contained.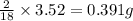 of hydrogen will be contained.
of hydrogen will be contained.![(4.30g)-[(2.34g)+(0.391g)]=1.57g](/tpl/images/0308/8464/24f5b.png)
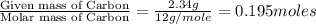
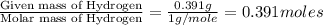
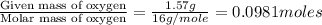
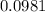 moles.
moles.