Chemistry, 11.10.2019 02:20 roneesmith2016
The expression of the theoretical yield (ty) in function of limiting reagent (lr) of a reaction is as follows: ty = ideal mole ratio of (target product / lr) x #mol(lr) x mw(target product) the ideal mole ratio is the one provided by the equation of the reaction. if a reaction uses (4.50x10^0) g of aniline and 1.25 times as many ml of acetic anhydride as the number of grams of aniline, what is the theoreticl yiled of acetanilide (mw = 135.17 g/mol) in the reaction?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 21.06.2019 22:30
Write the symbol for every chemical element that has atomic number greater than 3 and atomic mass less than 12.0 u.
Answers: 1


Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1
You know the right answer?
The expression of the theoretical yield (ty) in function of limiting reagent (lr) of a reaction is a...
Questions





Mathematics, 16.04.2021 02:20

Mathematics, 16.04.2021 02:20

Biology, 16.04.2021 02:20

Arts, 16.04.2021 02:20


Mathematics, 16.04.2021 02:20




Mathematics, 16.04.2021 02:20

Mathematics, 16.04.2021 02:20

Mathematics, 16.04.2021 02:20

English, 16.04.2021 02:20



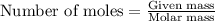 .....(1)
.....(1)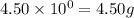 (We know that:
(We know that:  )
)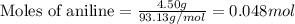
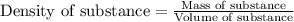
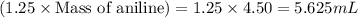
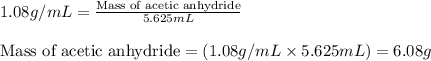
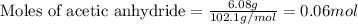

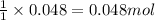 of acetic anhydride
of acetic anhydride