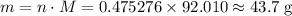50.0 g of n2o4(g) is placed in a 2.0 l evacuated flask and the system is allowed to reach equilibrium according to the reaction: n2o4(g) ⇄ 2 no2(g) kc = 0.133 after the system reaches equilibrium, 5.00 g of no2(g) is injected into the vessel, and the system is allowed to equilibrate once again. calculate the mass of n2o4 in the final equilibrium mixture.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2

Chemistry, 23.06.2019 01:00
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1

Chemistry, 23.06.2019 06:30
Achemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 2 no(g) + cl2(g) < => 2 nocl(g) kp = 2 x 10^(-6)he fills a reaction vessel at this temperature with 13. atm of nitrogen monoxide gas and 12. atm of chlorine gas. use this data to answer the questions: a. can you predict the equilibrium pressure of noci, using only the tools available to you within aleks? y/nb. if you said yes, then enter the equilibrium pressure of nocl at right. round your answer to 1 significant digit.
Answers: 1
You know the right answer?
50.0 g of n2o4(g) is placed in a 2.0 l evacuated flask and the system is allowed to reach equilibriu...
Questions

English, 28.10.2019 15:31



Mathematics, 28.10.2019 15:31

Computers and Technology, 28.10.2019 15:31



English, 28.10.2019 15:31









Chemistry, 28.10.2019 15:31

History, 28.10.2019 15:31

Mathematics, 28.10.2019 15:31

Geography, 28.10.2019 15:31

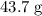 .
.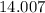 ;O:
;O: 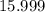 .
.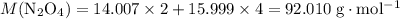 .
.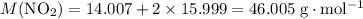 .
.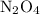 in that sample of
in that sample of 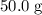 :
: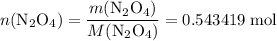 .
.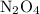 in that
in that 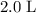 container:
container: 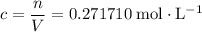 .
.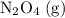 be
be 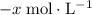 .
.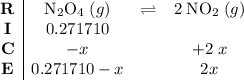 .
.![\displaystyle \frac{[\rm NO_2]^{2}}{[\rm N_2O_4]} = \rm K_{c} = 0.133](/tpl/images/0308/9684/7a7f2.png) .
.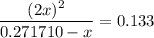 .
. .
. 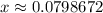 .
.![\rm [N_2O_4] \approx 0.271710 - 0.0798672 = \rm 0.191843 \;mol\cdot L^{-1}](/tpl/images/0308/9684/a5112.png) .
.![\rm [NO_2] \approx 2 \times 0.0798672 = 0.159734\; mol\cdot L^{-1}](/tpl/images/0308/9684/f286e.png) .
.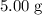 of
of ![\rm[NO_2]](/tpl/images/0308/9684/e223a.png) if it was added to an evacuated
if it was added to an evacuated 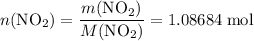 .
.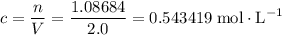 .
.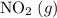 will become
will become 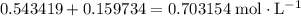 .
.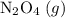 be
be 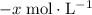 .
.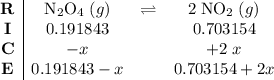 .
. 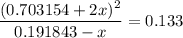 .
.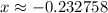 .
.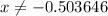 for if that would lead to a negative value for the concentration of
for if that would lead to a negative value for the concentration of 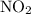 .
.![[{\rm N_2O_4}] = 0.703154 + 2(-0.232758) = \rm 0.237638\; mol\cdot L^{-1}](/tpl/images/0308/9684/8370c.png) .
.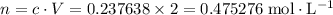 .
.