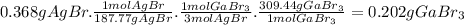Chemistry, 11.10.2019 02:30 iamabouttofail
Asolid mixture weighs 0.6813 g. it contains gallium bromide (gabr3) and other inert impurities. when the solid mixture was dissolved in water and treated with excess silver nitrate (agno3), 0.368 g of agbr was precipitate. a balanced chemical equation describing the reaction is provided below. gabr3(aq) + 3 agno3(aq) ⟶ 3 agbr(s) + ga(no3)3(aq) what is the percent of mass of gabr3 in the solid mixture?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is important to study for nios grade 12 chemistry? i have only one month left.
Answers: 2

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1
You know the right answer?
Asolid mixture weighs 0.6813 g. it contains gallium bromide (gabr3) and other inert impurities. when...
Questions


Geography, 15.04.2020 19:53




Mathematics, 15.04.2020 19:53

Social Studies, 15.04.2020 19:53





Mathematics, 15.04.2020 19:53

Mathematics, 15.04.2020 19:53

Mathematics, 15.04.2020 19:53



Geography, 15.04.2020 19:54