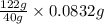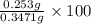An unknown amount of acid can often be determined by adding an excess of base and then back-titrating the excess. a 0.3471−g sample of a mixture of oxalic acid, which has two ionizable protons, and benzoic acid, which has one, is treated with 97.0 ml of 0.1090 m naoh. the excess naoh is titrated with 21.00 ml of 0.2060 m hcl. find the mass % of benzoic acid.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:50
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1

Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3

You know the right answer?
An unknown amount of acid can often be determined by adding an excess of base and then back-titratin...
Questions




















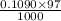
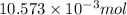
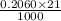
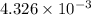 mol
mol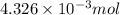
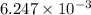 mol
mol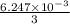
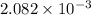 mol
mol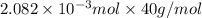
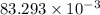 g
g