
Chromium has an atomic mass of 51.9961 u51.9961 u and consists of four isotopes, cr50,cr50, cr52,cr52, cr53,cr53, and cr54.cr54. the cr52cr52 isotope has a natural abundance of 83.79%83.79% and an atomic mass of 51.9405 u.51.9405 u. the cr54cr54 isotope has a natural abundance of 2.37%2.37% and an atomic mass of 53.9389 u.53.9389 u. the natural abundances of the cr50cr50 and cr53cr53 isotopes exist in a ratio of 0.4579: 1,0.4579: 1, and the cr50cr50 isotope has an atomic mass of 49.9460 u.49.9460 u. determine the atomic mass of the cr53 isotope. cr53 isotope.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3

Chemistry, 23.06.2019 05:20
Explain how global warming could have affected yellowstone frog and salamander habitat's, resulting in changes in the populations of these species
Answers: 2

Chemistry, 23.06.2019 07:00
0.88 moles of n2o5 (g) was placed in a sealed 1.00 l vessel. calculate the equilibrium concentration of n2o5. no2, and o2 and the equilibrium constant after equilibrium has been reached by 65.0% of the n2o5 decomposing.
Answers: 1
You know the right answer?
Chromium has an atomic mass of 51.9961 u51.9961 u and consists of four isotopes, cr50,cr50, cr52,cr5...
Questions



History, 06.12.2021 20:30

History, 06.12.2021 20:30

English, 06.12.2021 20:30








Mathematics, 06.12.2021 20:30





Mathematics, 06.12.2021 20:30


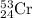 isotope is 52.8367 amu
isotope is 52.8367 amu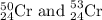 isotopes =
isotopes = ![[100-(83.79+2.37)]=13.84\%](/tpl/images/0309/0133/29a15.png)
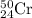 isotope =
isotope = 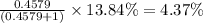
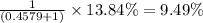
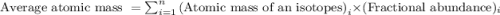 .....(1)
.....(1)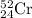 isotope:
isotope: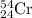 isotope:
isotope:![51.9961=[(49.9460\times 0.0437)+(51.9405\times 0.8379)+(x\times 0.0949)+(53.9389\times 0.0237)]\\\\x=52.8367amu](/tpl/images/0309/0133/822e6.png)


