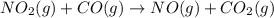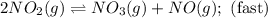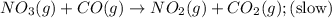Chemistry, 11.10.2019 04:00 aurelio1121
Nitrogen dioxide reacts with carbon monoxide to produce nitrogen monoxide and carbon dioxide. no2(g) + co(g) ⟶ no(g) + co2(g) a proposed mechanism for this reaction is 2no2(g) ⟶ no3(g) + no(g) (fast, equilibrium) no3(g) + co(g) ⟶ no2(g) + co2(g) (slow) what is a rate law that is consistent with the proposed mechanism?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1
You know the right answer?
Nitrogen dioxide reacts with carbon monoxide to produce nitrogen monoxide and carbon dioxide. no2(g)...
Questions

English, 05.02.2020 01:01

History, 05.02.2020 01:01

Chemistry, 05.02.2020 01:01




History, 05.02.2020 01:01

Mathematics, 05.02.2020 01:01


Arts, 05.02.2020 01:01

Mathematics, 05.02.2020 01:01

History, 05.02.2020 01:01








![\text{Rate}=k[NO_3][CO]](/tpl/images/0309/1117/3ba68.png)