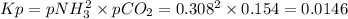nh4co2nh2< > 2nh3(g) + co2(g)


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1
You know the right answer?
Ammonium carbonate nh4co2nh2 decomposes as follows
nh4co2nh2< > 2nh3(g) + co2(g)
nh4co2nh2< > 2nh3(g) + co2(g)
Questions



Mathematics, 12.01.2021 17:30








History, 12.01.2021 17:30

Mathematics, 12.01.2021 17:30


Geography, 12.01.2021 17:30





History, 12.01.2021 17:30