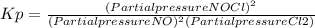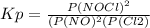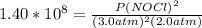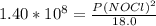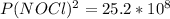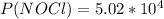Chemistry, 11.10.2019 17:30 BatmanVS1944
2 no(g) + cl2(g) ⇄ 2 nocl(g) kp = 1.40 × 108 a reaction vessel initially contains 3.0 atm of no and 2.0 atm of cl2(g). what is the pressure of no(g) when equilibrium is reached?
3.6 × 10-4 atm
3.8 × 10-8 atm
0.5 atm
2.0 atm
1.0 atm
1.1 × 10-7 atm

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2


Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3

Chemistry, 23.06.2019 09:00
How many grams of ammonia are produced when 1.0 mole of nitrogen reacts
Answers: 2
You know the right answer?
2 no(g) + cl2(g) ⇄ 2 nocl(g) kp = 1.40 × 108 a reaction vessel initially contains 3.0 atm of no...
Questions



Mathematics, 29.10.2020 03:10



Arts, 29.10.2020 03:10








Mathematics, 29.10.2020 03:10


Mathematics, 29.10.2020 03:10