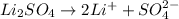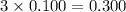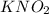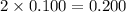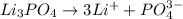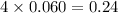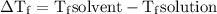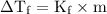Choose the aqueous solution that has the highest boiling point. these are all solutions of nonvolatile solutes and you should assume ideal van't hoff factors where applicable. choose the aqueous solution that has the highest boiling point. these are all solutions of nonvolatile solutes and you should assume ideal van't hoff factors where applicable. a. 0.100 m li₂so₄
b. 0.100 m kno₂
c. 0.200 m c₃h₈o₃
d. 0.060 m li₃po₄e. they all have the same boiling point.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Which of the following best describes the formation of plasma?
Answers: 1


Chemistry, 23.06.2019 01:00
The primary products of complete combustion of fossil fuels are a. carbon dioxide and water b. methane and water c. carbon monoxide and water d. carbon dioxide and carbon monoxide
Answers: 1

Chemistry, 23.06.2019 01:30
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3
You know the right answer?
Choose the aqueous solution that has the highest boiling point. these are all solutions of nonvolati...
Questions

Biology, 02.08.2019 20:00


Mathematics, 02.08.2019 20:00


History, 02.08.2019 20:00

History, 02.08.2019 20:00

Mathematics, 02.08.2019 20:00







Mathematics, 02.08.2019 20:00






Mathematics, 02.08.2019 20:00


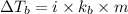
 = Elevation in boiling point
= Elevation in boiling point  = boiling point constant
= boiling point constant