
Chemistry, 11.10.2019 23:10 erniewernie
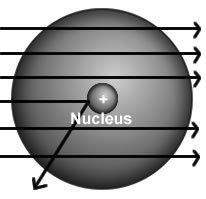
The results of rutherford's gold foil experiment gave him the evidence to arrive at two conclusions: (1) an atom was much more than just empty space and scattered electrons and (2)
a) in any atom, electrons orbit a central nucleus.
b) all atoms were composed of three subatomic particles.
c) an atom must have a positively charged center that contains most of its mass.
d) an atom must have an equal number of positive and negative particles in a central location.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Aroller coaster car is traveling down a track at 22 m/s. the car has a mass of 2000 kg. what is the kinetic energy of the car? a) 22,000 j b) 968,000 j c) 484,000 j d) 44,000 j
Answers: 2

Chemistry, 22.06.2019 03:20
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1
You know the right answer?
The results of rutherford's gold foil experiment gave him the evidence to arrive at two conclusions:...
Questions


Biology, 16.04.2021 22:20

Mathematics, 16.04.2021 22:20


Biology, 16.04.2021 22:20

Mathematics, 16.04.2021 22:20


Mathematics, 16.04.2021 22:20




Chemistry, 16.04.2021 22:20

Mathematics, 16.04.2021 22:20



Mathematics, 16.04.2021 22:20



Mathematics, 16.04.2021 22:20

History, 16.04.2021 22:20



