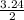Chemistry, 11.10.2019 23:30 redthangracing
2.00 mol of h2(g) and 1.00 mol of i2(g) are placed in a 1.00 l container, and they react to form hi(g). at equilibrium, it is found that 1.80 moles of hi(g) are present in the container. calculate k for the reaction: h2(g) i2(g) ⇄ 2 hi(g)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1
You know the right answer?
2.00 mol of h2(g) and 1.00 mol of i2(g) are placed in a 1.00 l container, and they react to form hi(...
Questions


Mathematics, 05.12.2021 23:40

Biology, 05.12.2021 23:40

History, 05.12.2021 23:40

Chemistry, 05.12.2021 23:40


Mathematics, 05.12.2021 23:40

Mathematics, 05.12.2021 23:40

Mathematics, 05.12.2021 23:40

English, 05.12.2021 23:40





English, 05.12.2021 23:40


Mathematics, 05.12.2021 23:40


Chemistry, 05.12.2021 23:40

Mathematics, 05.12.2021 23:40

![k = \frac{[[HI]^{2} }{[H2][I2]}](/tpl/images/0311/6358/eab06.png)
![k = \frac{[1.8]^{2} }{[2][1]}](/tpl/images/0311/6358/ea4d9.png)