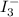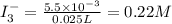The amount of i₃⁻(aq) in a solution can be determined by titration with a solution containing a known concentration of s₂o₂⁻³(aq) (thiosulfate ion). the determination is based on the net ionic equation 2s₂o₃²⁻(aq)+i₃⁻(aq)⟶s₄o₆²⁻(aq)+3i⁻( aq). given that it requires 38.1 ml of 0.440 m na₂s₂o₃(aq) to titrate a 25.0 ml sample of i₃⁻(aq), calculate the molarity of i₃⁻(aq) in the solution.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2
You know the right answer?
The amount of i₃⁻(aq) in a solution can be determined by titration with a solution containing a know...
Questions







Advanced Placement (AP), 05.10.2019 09:01


Mathematics, 05.10.2019 09:01

History, 05.10.2019 09:01

English, 05.10.2019 09:01


Mathematics, 05.10.2019 09:01


Computers and Technology, 05.10.2019 09:01


Biology, 05.10.2019 09:01



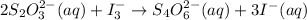
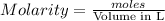
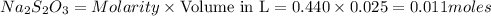
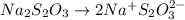
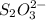 = 0.011
= 0.011 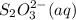 require 1 mole of
require 1 mole of 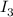
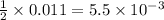 moles of
moles of