Which net ionic equation would match this description of a chemical reaction: when a
solution...

Chemistry, 12.10.2019 01:20 michaellagann2020
Which net ionic equation would match this description of a chemical reaction: when a
solution of magnesium nitrate is mixed with a solution of sodium phosphate, the solid that forms is magnesium phosphate.
a. mg(no3)2(aq) + na2po4(aq) à mgpo4(s) + nano3(aq)
b. mg2+(aq) + po43-(aq) à mgpo4(s)
c. 3mg(no3)2(aq) + 2na3po4(aq) à mg3(po4)2(s) + 6nano3(aq)
d. 3mg2+(aq) + 2po43- à mg3(po4)2(s)
e. 3mg2+(aq) + 6no3-(aq) + 6na+(aq) + 2po43- àmg3(po4)2(s) + 6no3-(aq) + 6na+(aq)
it shuld be d correct?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3


Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3
You know the right answer?
Questions

English, 15.11.2019 19:31


Mathematics, 15.11.2019 19:31

Mathematics, 15.11.2019 19:31




Spanish, 15.11.2019 19:31




Social Studies, 15.11.2019 19:31

Mathematics, 15.11.2019 19:31


History, 15.11.2019 19:31

History, 15.11.2019 19:31




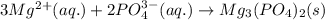
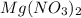 ,
, 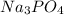 are strong electrolytes. Hence they are fully ionized in aqueous solution.
are strong electrolytes. Hence they are fully ionized in aqueous solution.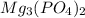 is a sparingly soluble salt. Hence it remains undissociated in aqueous solution.
is a sparingly soluble salt. Hence it remains undissociated in aqueous solution.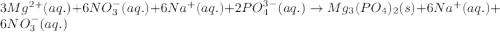
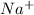 and
and 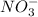 ions are spectator ions as they remain present on both side of total ionic equation.
ions are spectator ions as they remain present on both side of total ionic equation.

