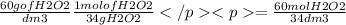Chemistry, 12.10.2019 19:30 kevonmajor
Hydrogen peroxide is sold commercially as an aqueous solution containing approximately 60 g/dm³ of hyrogen peroxide.
calculate the concentration in mol/dm³, of a solution containing 60.0 g/dm³ of hydrogen peroxide.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 02:30
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

You know the right answer?
Hydrogen peroxide is sold commercially as an aqueous solution containing approximately 60 g/dm³ of h...
Questions



Social Studies, 12.07.2019 22:30


History, 12.07.2019 22:30

Business, 12.07.2019 22:30


Business, 12.07.2019 22:30


Social Studies, 12.07.2019 22:30

Biology, 12.07.2019 22:30




Social Studies, 12.07.2019 22:30


History, 12.07.2019 22:30

History, 12.07.2019 22:30


Business, 12.07.2019 22:30