Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2


Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2
You know the right answer?
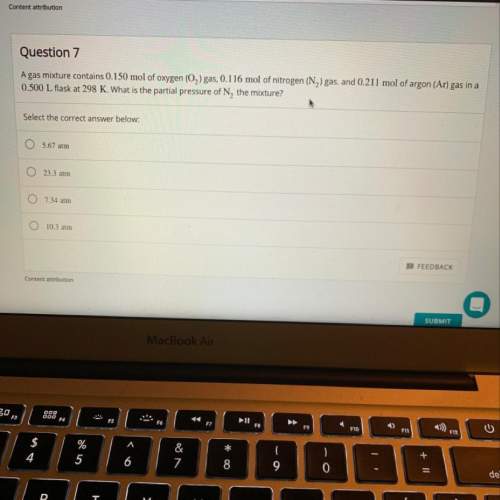
Agas mixture contains 0.150 mol of o2 gas, 0.116 mol of n2 gas, and 0.211 mol of ar gas in a 0.500 l...
Questions

English, 27.04.2021 20:40

Chemistry, 27.04.2021 20:40


Mathematics, 27.04.2021 20:40


History, 27.04.2021 20:40

History, 27.04.2021 20:40

History, 27.04.2021 20:40

Mathematics, 27.04.2021 20:40



Mathematics, 27.04.2021 20:40

Mathematics, 27.04.2021 20:40

Mathematics, 27.04.2021 20:40

Mathematics, 27.04.2021 20:40

History, 27.04.2021 20:40

Biology, 27.04.2021 20:40



Physics, 27.04.2021 20:40