
Chemistry, 14.10.2019 17:30 alexthebest3976
Which of the following chemical equations does not correspond to a standard molar enthalpy of formation? a. mg(s) + c(s) + 3/2 o_2(g) rightarrow mgco_3(s) b. c(s) + 1/2 o_2(g) rightarrow co(g) c. n_2(g) + o_2(g) rightarrow 2 no(g) d. n_2(g) + 2 o_2(g) rightarrow n_2o_4(g) e. h_2(g)+l/2 o_2(g) rightarrow h_2o(l)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Write a paragraph at least 10 sentences explaining how rocks are used today in society
Answers: 1

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 22.06.2019 17:20
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2
You know the right answer?
Which of the following chemical equations does not correspond to a standard molar enthalpy of format...
Questions

Mathematics, 06.12.2021 17:50






Biology, 06.12.2021 17:50


French, 06.12.2021 17:50

Arts, 06.12.2021 17:50

Mathematics, 06.12.2021 17:50


Arts, 06.12.2021 17:50

World Languages, 06.12.2021 17:50


Social Studies, 06.12.2021 17:50


Mathematics, 06.12.2021 17:50

Mathematics, 06.12.2021 17:50

 + O₂
+ O₂ → 2NO
→ 2NO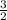 O
O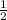 O₂
O₂


