
Chemistry, 14.10.2019 19:00 Tristanana
Consider the equilibrium reaction: 3cio-(aq) ↔ cio3-(aq) + 2ci-(aq) the equilibrium constant kc = 3.2 x 103. the following concentrations are present: [cl-] = 0.50 mol/l; [clo3-] = 0.32 mol/l; [clo-] = 0.24 mol/l. is the mixture at equilibrium and, if not, in which direction will reaction proceed?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2


Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 23.06.2019 00:30
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1
You know the right answer?
Consider the equilibrium reaction: 3cio-(aq) ↔ cio3-(aq) + 2ci-(aq) the equilibrium constant kc = 3...
Questions

Mathematics, 05.11.2020 06:40

Mathematics, 05.11.2020 06:40


Chemistry, 05.11.2020 06:40

Mathematics, 05.11.2020 06:40


Health, 05.11.2020 06:40

Mathematics, 05.11.2020 06:40


Physics, 05.11.2020 06:40


Mathematics, 05.11.2020 06:40

Chemistry, 05.11.2020 06:40

Mathematics, 05.11.2020 06:40


Mathematics, 05.11.2020 06:40


History, 05.11.2020 06:40


History, 05.11.2020 06:40

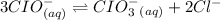
![Q=\frac {[CIO_3^{-}][Cl^{-}]^2}{[CIO^{-}]^3}](/tpl/images/0319/2491/848d4.png)
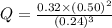
 )
)

