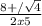Chemistry, 14.10.2019 21:00 missdee6929
H2(g) + i2(g) â 2 hi(g) kc = 64 at 400âºc 3.00 moles of h2 and 3.00 moles of i2 are placed in a 4.00 l container and the system is allowed to reach equilibrium. calculate the concentration of hi at equilibrium.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:10
Agas mixture with a total pressure of 745 mmhg contains each of the following gases at the indicated partial pressures: co2, 245 mmhg ; ar, 119 mmhg ; and o2, 163 mmhg . the mixture also contains helium gas. part a what is the partial pressure of the helium gas? phe p h e = nothing mmhg request answer part b what mass of helium gas is present in a 10.2-l sample of this mixture at 283 k ? m m = nothing g request answer
Answers: 1

Chemistry, 21.06.2019 22:10
Here’s one way to follow the scientific method. place the missing steps in the correct position in the process
Answers: 1

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1
You know the right answer?
H2(g) + i2(g) â 2 hi(g) kc = 64 at 400âºc 3.00 moles of h2 and 3.00 moles of i2 are placed in a 4.00...
Questions

Mathematics, 28.05.2020 16:57

Mathematics, 28.05.2020 16:57

History, 28.05.2020 16:57









English, 28.05.2020 16:57

Mathematics, 28.05.2020 16:57

Mathematics, 28.05.2020 16:57





Mathematics, 28.05.2020 16:57

![Kc = \frac{{D}^dx[C]^c}{[A]^ax[B]^b}](/tpl/images/0319/5603/47fdd.png)
![Kc = \frac{[HI]^2}{[H2]x[I2]}](/tpl/images/0319/5603/fe065.png)