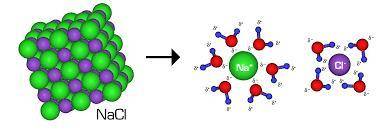
How does dissolving an ionic compound in water increase electrical conductivity?
a. the...

Chemistry, 15.10.2019 00:00 hilzepesqtatiana
How does dissolving an ionic compound in water increase electrical conductivity?
a. the water molecules electrostatically interact with the ions in the crystal and let the ions move freely.
b. the water molecules apply heat to break the ionic bonds in the crystal and let the ions move freely.
c. the water molecules chemically react with the ions in the crystal and let the ions move freely.
d. the water molecules remove heat to break the ionic bonds in the crystal and let the ions move freely.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1


Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2
You know the right answer?
Questions



Mathematics, 17.09.2019 02:00


Mathematics, 17.09.2019 02:00

Mathematics, 17.09.2019 02:00

History, 17.09.2019 02:00


Engineering, 17.09.2019 02:00

English, 17.09.2019 02:00

History, 17.09.2019 02:00

Mathematics, 17.09.2019 02:00

Social Studies, 17.09.2019 02:00

History, 17.09.2019 02:00

English, 17.09.2019 02:00

Biology, 17.09.2019 02:00




English, 17.09.2019 02:00