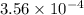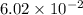Ammonia can be produced via the chemicalreaction
\rm n_2 (g) + 3 h_2 (g) \rightleftharpoons 2 nh_3 (g)
during the production process, the production engineer determinesthe reaction quotient to be q_2 = 3.56ã10â4. ifk_2 = 6.02ã10â2, what can besaid about the reaction?
1)the reaction has reached equilibrium.
2)the reaction is not at equilibrium and willproceed to the left.
3)the reaction is not at equilibrium and willproceed to the right.
4)the reaction is not at equilibrium, but it isnot possible to determine whether the reaction needs to proceedright or left to reach equilibrium.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 23:00
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1

Chemistry, 22.06.2019 23:10
Amines are good nucleophiles, even though they are neutral molecules. how would the rate of an sn2 reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? amines are good nucleophiles, even though they are neutral molecules. how would the rate of an reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? because both reactants in the rate-limiting step are neutral, the reaction will be faster if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will be slower if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will occur at the same rate if the polarity of the solvent is increased. request answer
Answers: 3

Chemistry, 23.06.2019 04:31
How does a sample of helium at 15 degree celsius compare to a sample of helium at 215 k? a) the helium at 15 degrees celsius has a higher average kinetic energy that the sample at 215 k. b) the helium at 15 degrees celsius has lower nuclear energy that the sample at 215 k. c) the helium at 15 degrees celsius has slower- moving atoms that the sample at 215 k. d) the helium at 15 degrees celsius has smaller atoms than the sample at 215 k.
Answers: 1
You know the right answer?
Ammonia can be produced via the chemicalreaction
\rm n_2 (g) + 3 h_2 (g) \rightleftharpoons 2...
\rm n_2 (g) + 3 h_2 (g) \rightleftharpoons 2...
Questions

Arts, 01.02.2021 22:40





Mathematics, 01.02.2021 22:40

Biology, 01.02.2021 22:40


Mathematics, 01.02.2021 22:40

History, 01.02.2021 22:40


Chemistry, 01.02.2021 22:40

Mathematics, 01.02.2021 22:40

Mathematics, 01.02.2021 22:40


Mathematics, 01.02.2021 22:40


History, 01.02.2021 22:40


History, 01.02.2021 22:40