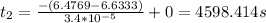At 25∘c, the decomposition of dinitrogen pentoxide, n2o5(g), into no2(g) and o2(g) follows first-order kinetics with k=3.4×10−5 s−1. a sample of n2o5 with an initial pressure of 760 torr decomposes at 25∘c until its partial pressure is 650 torr. how much time (in seconds) has elapsed? at 25, the decomposition of dinitrogen pentoxide, (g), into (g) and (g) follows first-order kinetics with . a sample of with an initial pressure of 760 decomposes at 25 until its partial pressure is 650 . how much time (in seconds) has elapsed? 5.3×10−6 2000 4600 34,000 190,000

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 19:00
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1

Chemistry, 23.06.2019 00:30
Which of the following best describes technology a. something created for only scientists to use b.the method of thinking that scientists use. c.the application of engineering to create useful products. c. a scientific idea
Answers: 1
You know the right answer?
At 25∘c, the decomposition of dinitrogen pentoxide, n2o5(g), into no2(g) and o2(g) follows first-ord...
Questions

History, 10.09.2019 19:30


Computers and Technology, 10.09.2019 19:30



Mathematics, 10.09.2019 19:30

Mathematics, 10.09.2019 19:30

History, 10.09.2019 19:30


Mathematics, 10.09.2019 19:30


Biology, 10.09.2019 19:30

Mathematics, 10.09.2019 19:30


Mathematics, 10.09.2019 19:30



Social Studies, 10.09.2019 19:30

History, 10.09.2019 19:30

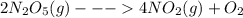
![-\frac{d[B]}{dt}=k[B] - - - -\frac{d[B]}{[B]}=k*dt](/tpl/images/0320/7058/5f6ff.png)
![-\frac{d[P(B)]}{P(B)}=k*dt](/tpl/images/0320/7058/d243b.png)
![-\frac{d[P(N_{2}O_{5})]}{P(N_{2}O_{5})}=k*dt](/tpl/images/0320/7058/282a3.png)
![\int\limits^p \,-\frac{d[P(N_{2}O_{5})]}{P(N_{2}O_{5})}=\int\limits^ t k*dt](/tpl/images/0320/7058/1b0c7.png)
![-(ln[P(N_{2}O_{5})]-ln[P(N_{2}O_{5})_{o})])=k(t_{2}-t_{1})](/tpl/images/0320/7058/3450d.png)
![\frac{-(ln[P(N_{2}O_{5})]-ln[P(N_{2}O_{5})_{o})])}{k}+t_{1}=t_{2}](/tpl/images/0320/7058/b0e0f.png)
![ln[P(N_{2}O_{5})]=ln(650)=6.4769](/tpl/images/0320/7058/0a747.png)
![ln[P(N_{2}O_{5})_{o}]=ln(760)=6.6333](/tpl/images/0320/7058/11e7e.png)