
Chemistry, 15.10.2019 04:00 johnnyboy41706
For each of the following unbalanced equations, calculate how many grams of each product would be produced by complete reaction of 10.8 g of the second reactant. (a) s(s) + h2so4(aq) → so2(g) + h2o(l) so2 webassign will check your answer for the correct number of significant figures. g h2o webassign will check your answer for the correct number of significant figures. g

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 02:30
24 points and brainliest to anyone who can answer under 10 minutes with best ! the table below shows the role of different substances during photosynthesis. substance role during photosynthesis glucose stores chemical energy water combines with glucose to form carbon dioxide chlorophyll traps sunlight which of the following statements would correct one of the roles listed in the table? glucose combines with carbon to form water. chlorophyll reacts with light to produce carbon dioxide. water combines with carbon dioxide during photosynthesis. chlorophyll stores chemical energy needed for photosynthesis.
Answers: 1


Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1
You know the right answer?
For each of the following unbalanced equations, calculate how many grams of each product would be pr...
Questions

Mathematics, 29.09.2020 17:01



Mathematics, 29.09.2020 17:01





English, 29.09.2020 17:01

History, 29.09.2020 17:01




English, 29.09.2020 17:01

Social Studies, 29.09.2020 17:01

Chemistry, 29.09.2020 17:01




 of
of 
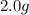 of
of 





