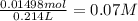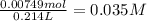Chemistry, 15.10.2019 04:20 madams1820
A) how many grams of silver chloride can be prepared by the reaction of 107.0 ml of 0.21 m silver nitrate with 107.0 ml of 0.14 m calcium chloride?
g
b) calculate the concentrations of each ion remaining in solution after precipitation is complete.
ag+ m
no3? m
ca2+ m
cl? m

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3
You know the right answer?
A) how many grams of silver chloride can be prepared by the reaction of 107.0 ml of 0.21 m silver ni...
Questions

Biology, 29.08.2019 09:50


History, 29.08.2019 09:50




Mathematics, 29.08.2019 09:50


Chemistry, 29.08.2019 09:50

Biology, 29.08.2019 09:50


English, 29.08.2019 09:50

History, 29.08.2019 09:50





Social Studies, 29.08.2019 09:50

Business, 29.08.2019 09:50

Business, 29.08.2019 09:50


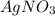
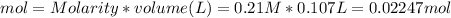
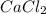
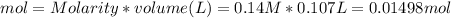
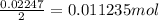
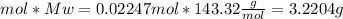
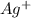 since we used all the
since we used all the 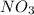 this ion was used to produce
this ion was used to produce 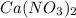 and this is soluble in water. The moles of
and this is soluble in water. The moles of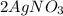 before the reaction because this ion did not precipitate but it's concentration change because the volume changed.
before the reaction because this ion did not precipitate but it's concentration change because the volume changed. 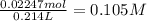
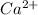 did not precipitated so we have the same number of moles of
did not precipitated so we have the same number of moles of