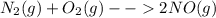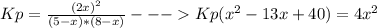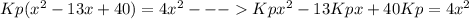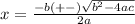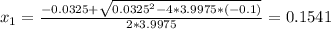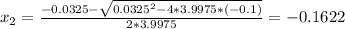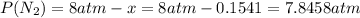Chemistry, 15.10.2019 05:30 elizabethburkha
Consider the reaction n2(g) + o2(g) â 2 no(g) k = 0.0025 a rigid container initially contains 8.00 atm of nitrogen and 5.00 atm of oxygen. what will be the partial pressure of nitrogen at equilibrium?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1


Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2
You know the right answer?
Consider the reaction n2(g) + o2(g) â 2 no(g) k = 0.0025 a rigid container initially contains 8.00 a...
Questions

Computers and Technology, 07.01.2021 21:00

English, 07.01.2021 21:00


English, 07.01.2021 21:00


History, 07.01.2021 21:00



Physics, 07.01.2021 21:00

Advanced Placement (AP), 07.01.2021 21:00



Computers and Technology, 07.01.2021 21:00

Mathematics, 07.01.2021 21:00


Biology, 07.01.2021 21:00

Health, 07.01.2021 21:00


Geography, 07.01.2021 21:00

Mathematics, 07.01.2021 21:00