
Chemistry, 15.10.2019 07:10 xxaurorabluexx
Gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . suppose 2.3 g of butane is mixed with 15.0 g of oxygen. calculate the minimum mass of butane that could be left over by the chemical reaction. be sure your answer has the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Which term refers to a property that depends only on the amount of a substance? ©@
Answers: 2

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1
You know the right answer?
Gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water ....
Questions

Biology, 22.01.2020 06:32

Mathematics, 22.01.2020 06:32

Social Studies, 22.01.2020 06:32

Mathematics, 22.01.2020 06:32


English, 22.01.2020 06:32

Mathematics, 22.01.2020 06:32



English, 22.01.2020 06:32


Mathematics, 22.01.2020 06:32

Mathematics, 22.01.2020 06:32

Mathematics, 22.01.2020 06:32




Mathematics, 22.01.2020 06:32

Mathematics, 22.01.2020 06:32

Mathematics, 22.01.2020 06:32

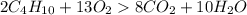
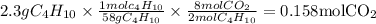
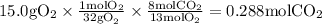
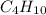 is the Limiting reactant and
is the Limiting reactant and 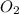 is the excess reactant
is the excess reactant
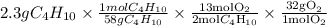
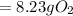 is the actual need
is the actual need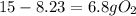 is left unreacted (Answer)
is left unreacted (Answer)


