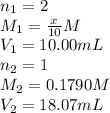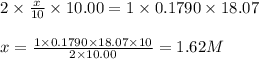Chemistry, 15.10.2019 18:00 kingalex7575
Achemist needs to determine the concentration of a sulfuric acid solution by titration with a standard sodium hydroxide solution. he has a 0.1790 m standard sodium hydroxide solution. he takes a 25.00 ml sample of the original acid solution and dilutes it to 250.0 ml. then, he takes a 10.00 ml sample of the dilute acid solution and titrates it with the standard solution. the endpoint was reached after the addition of 18.07 ml of the standard solution. what is the concentration of the original sulfuric acid solution?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2


Chemistry, 23.06.2019 02:00
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1
You know the right answer?
Achemist needs to determine the concentration of a sulfuric acid solution by titration with a standa...
Questions












Mathematics, 06.11.2019 03:31





History, 06.11.2019 03:31



Mathematics, 06.11.2019 03:31

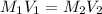
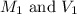 are the molarity and volume of the concentrated sulfuric acid solution
are the molarity and volume of the concentrated sulfuric acid solution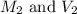 are the molarity and volume of diluted sulfuric acid solution
are the molarity and volume of diluted sulfuric acid solution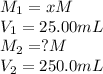
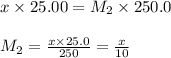
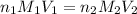
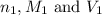 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 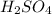
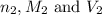 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.