

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1

Chemistry, 23.06.2019 00:30
You are attempting to recrystallize a crude product mixture. you add the appropriate amount of hot solvent and are allowing the solution to slowly cool to room temperature. however, at room temperature no crystals have appeared, which of the following methods should be used to induce crystallization? choose all correct answers. a) place the flask in an ice bath. b) swirl the contents of the flask. c) add a small seed crystal of the desired product. d) scratch the inside of the glassware using a stir rod. it can be multiple choices
Answers: 3

Chemistry, 23.06.2019 05:30
Stoichiometry- i need with 14 and 15! an explanation would be appreciated!
Answers: 1
You know the right answer?
The approximate concentration of the naoh solution you will prepare in part a of this experiment is...
Questions


Chemistry, 30.10.2020 17:40


Spanish, 30.10.2020 17:40

Chemistry, 30.10.2020 17:40

History, 30.10.2020 17:40

History, 30.10.2020 17:40








Mathematics, 30.10.2020 17:40

Mathematics, 30.10.2020 17:40

Spanish, 30.10.2020 17:40


Geography, 30.10.2020 17:40

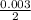 = 0.0015moles of oxalic acid
= 0.0015moles of oxalic acid

