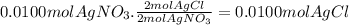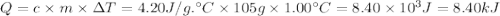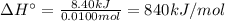Chemistry, 15.10.2019 20:30 coryintheswamp
10. when 50.0 ml of 0.200 m agno3 and 50.0 ml of 0.100 m cacl2, both at 25.0°c, are reacted in a coffee-cup calorimeter, the temperature of the reacting mixture increases to 26.0°c. calculate ∆h in kj per mole of agcl produced. assume the density of the solution is 1.05 g/ml and the specific heat capacity of the solution 4.20 j/g°c. agno3(aq) + hcl(aq) à agcl(s) + hno3(aq)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2
You know the right answer?
10. when 50.0 ml of 0.200 m agno3 and 50.0 ml of 0.100 m cacl2, both at 25.0°c, are reacted in a cof...
Questions


Mathematics, 01.03.2021 01:00

English, 01.03.2021 01:00




Mathematics, 01.03.2021 01:00

Mathematics, 01.03.2021 01:00

History, 01.03.2021 01:00


Biology, 01.03.2021 01:00

Mathematics, 01.03.2021 01:00


Mathematics, 01.03.2021 01:00


Mathematics, 01.03.2021 01:00

Social Studies, 01.03.2021 01:00

Mathematics, 01.03.2021 01:00

Chemistry, 01.03.2021 01:00