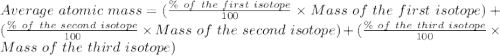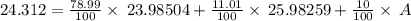Chemistry, 15.10.2019 21:20 francisco42002
Naturally occurring magnesium has an atomic mass of 24.312 and consists of three isotopes. the major isotope is mg24, natural abundance 78.99%, relative atomic mass 23.98504. the next most abundant isotope is mg26, relative atomic mass 25.98259. the third most abundant isotope is mg25 whose natural abundance is in the ratio of 0.9083 to that of mg26. find the relative atomic mass of mg25.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2


Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2
You know the right answer?
Naturally occurring magnesium has an atomic mass of 24.312 and consists of three isotopes. the major...
Questions

Mathematics, 29.06.2021 18:10




Mathematics, 29.06.2021 18:10

Mathematics, 29.06.2021 18:10

Mathematics, 29.06.2021 18:10


English, 29.06.2021 18:10




English, 29.06.2021 18:10


Mathematics, 29.06.2021 18:10



English, 29.06.2021 18:10