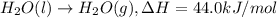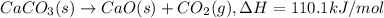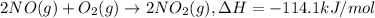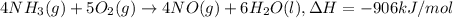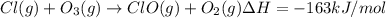Chemistry, 15.10.2019 22:00 Ashley606hernandez
Select whether there is no work done by the system, work done by the system or work done by the surroundings for each system described below:
a. h2o (l) ⟶ h2o (g) δh = 44.0 kj/mol b. 2 no (g) + o2 (g) ⟶ 2 no2(g) δh = -114.1 kj/mol c. cl (g) + o3 (g) ⟶ clo(g) + o2(g) δh = -163 kj/mol d. caco3 (s) ⟶ cao (s) + co2(g) δh = 110.1 kj/mol e. 4 nh3(g) + 5 o2(g) ⟶ 4 no (g) + 6 h2o(l) δh = -906 kj/mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1
You know the right answer?
Select whether there is no work done by the system, work done by the system or work done by the surr...
Questions



Mathematics, 05.01.2021 21:30

Arts, 05.01.2021 21:30

Mathematics, 05.01.2021 21:30



Mathematics, 05.01.2021 21:30

Geography, 05.01.2021 21:30



Mathematics, 05.01.2021 21:30



English, 05.01.2021 21:30




English, 05.01.2021 21:30

Mathematics, 05.01.2021 21:30