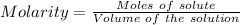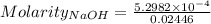Chemistry, 16.10.2019 01:30 jakails828
The concentration of a certain sodium hydroxide solution was determined by using the solution to titrate a sample of potassium hydrogen phthalate (abbreviated as khp). khp is an acid with one acidic hydrogen and a molar mass of 204.22 g/mol. in the titration, 24.46 ml of the sodium hydroxide solution was required to react with 0.1082 g khp. calculate the molarity of the sodium hydroxide.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1
You know the right answer?
The concentration of a certain sodium hydroxide solution was determined by using the solution to tit...
Questions


Mathematics, 06.05.2020 23:10



Mathematics, 06.05.2020 23:10

Mathematics, 06.05.2020 23:10




Computers and Technology, 06.05.2020 23:10

Chemistry, 06.05.2020 23:10


Biology, 06.05.2020 23:10







Mathematics, 06.05.2020 23:10

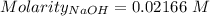
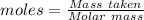
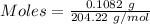
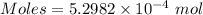
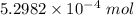 of KHP reacts with
of KHP reacts with