
Naturally occurring silicon has an atomic mass of 28.086 and consists of three isotopes. the major isotope is 28si, natural abundance 92.23%, relative atomic mass 27.97693. the next most abundant isotope is 29si, relative atomic mass 28.97649. the third isotope is 30si whose natural abundance is in the ratio of 0.6592 to that of 29si.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
One mole of zinc has a mass of 65.4 grams. approximately how many atoms of zinc are present in one mole of zinc? 32 × 1023 atoms 6 × 1023 atoms 66 atoms 65 atoms
Answers: 1

Chemistry, 21.06.2019 22:00
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2

Chemistry, 22.06.2019 03:00
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1
You know the right answer?
Naturally occurring silicon has an atomic mass of 28.086 and consists of three isotopes. the major i...
Questions

Spanish, 22.10.2020 21:01


Mathematics, 22.10.2020 21:01

Biology, 22.10.2020 21:01


Mathematics, 22.10.2020 21:01

Chemistry, 22.10.2020 21:01

Mathematics, 22.10.2020 21:01

Chemistry, 22.10.2020 21:01

Health, 22.10.2020 21:01

Advanced Placement (AP), 22.10.2020 21:01


History, 22.10.2020 21:01



Mathematics, 22.10.2020 21:01

Chemistry, 22.10.2020 21:01

Mathematics, 22.10.2020 21:01


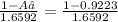 =0.0468
=0.0468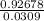 =Si³⁰
=Si³⁰

